What is Nitrite and
Why Is It in My Water?
Nitrites are a salt or ester anion of nitrous acid, which can be naturally or artificially occurring in groundwater. Nitrites come from fertilizers through run-off water, sewage, and mineral deposits. Nitrite is used in food production for the curing of meat products due to it inhibiting the growth of bacteria. Unfortunately it can also stimulate the grown of bacteria when introduced in high levels into a body of water.
What are the Health Effects of Nitrite Exposure?
High levels of nitrites are toxic to humans and animals, especially infants. It can enter the body as nitrate, a nutrient which is essential to plant growth, and be converted into nitrite, which disrupts the oxygen delivering ability of hemoglobin in the bloodstream. Infants can develop a life threatening blood disorder known as blue baby syndrome (methemoglobinemia) if exposed to it in water or formula mixed with water that is contaminated with nitrate.
What does Eutrophication, Hypoxia & Cyanobacteria have to do with Nitrite?
Eutrophication happens when artificial or natural substances are introduced into waterways, like nitrates and nitrites, from fertilizers (farm run-off) or untreated sewage. Large blooms of algae (cyanobacteria) can grow due the increased levels of nutrients in the water, which can lead to hypoxia. Another effect is decreased biodiversity in an ecosystem.
Hypoxia is a severe depletion of oxygen in a body of water which can be caused naturally or artificially, by phytoplankton (algae) blooms or freshwater flowing into a less dense body of water. These hypoxia ‘dead zones’ are deadly to almost all marine plant and animal life.

Cyanobacteria (phytoplankton or ‘blue-green algae’) get their energy the same way that plants do, through photosynthesis (sunlight). Excess nitrogen and phosphate nutrients cause increased and rapid growth of algae in a body of water. These bacteria consume oxygen in a body of water at night and when they die they drift to the floor and are consumed by other bacteria which also deplete oxygen in the water, which can create a dead zone. Some phytoplankton blooms can produce toxic compounds that, when consumed by livestock can move up the food chain and pose a threat to human health.
Microcystin is a toxic peptide is produced in large quantities when cyanobacteria blooms. This can affect drinking water greatly, (even boiling to purify it is not recommended, as it concentrates the toxins rather than removing them). On August 2nd, 2014 in Toledo Ohio, which draws it’s water from Lake Erie, detected microcystins which affected the water supply of 500,000 people.
Anatoxin-a is a cyanotoxin produced by certain types of cyanobacteria that has been referred to as Very Fast Death Factor due to its acute neurotoxicity. Discorvered in the 1960’s, this deadly toxin has been found in North America, Europe, Africa, Asia, and New Zealand. Typical methods of treating water, such as boiling, UV disinfection and chlorination, are not effective for the removal of Anatoxin-a.
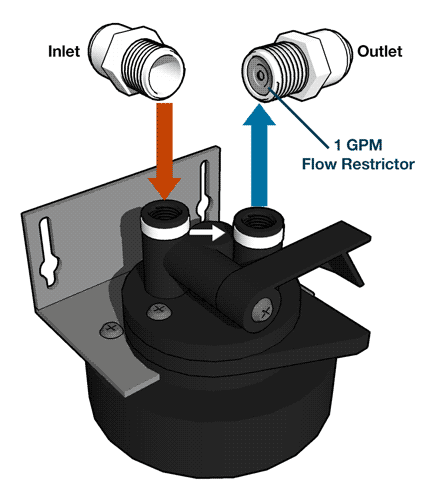
Nitrite Removal From Drinking Water
Reverse osmosis systems can remove nitrites from water with a thin-film membrane, a process known as ion exchange.
Reverse Osmosis Water Filters
NSF 53, 58 or
62 Certified
From NSF.org
NSF/ANSI Standard 53: Drinking Water Treatment Units – Health Effects
Overview: Standard 53 addresses point-of-use (POU) and point-of-entry (POE) systems designed to reduce specific health-related contaminants, such as Cryptosporidium, Giardia, lead, volatile organic chemicals (VOCs), MTBE (methyl tertiary-butyl ether), that may be present in public or private drinking water.
NSF/ANSI Standard 58: Reverse Osmosis Drinking Water Treatment Systems
Overview: This standard was developed for point-of-use (POU) reverse osmosis (RO) treatment systems. These systems typically consist of a pre-filter, RO membrane, and post-filter. Standard 58 includes contaminant reduction claims commonly treated using RO, including fluoride, hexavalent and trivalent chromium, total dissolved solids, nitrates, etc. that may be present in public or private drinking water.
NSF/ANSI Standard 62: Drinking Water Distillation Systems
Overview: Standard 62 covers distillation systems designed to reduce specific contaminants, including total arsenic, chromium, mercury, nitrate/nitrite, and microorganisms from public and private water supplies.
Sources of Information on Nitrite
- EPA – Nitrite in Drinking Water
- WHO – Nitrite in Drinking Water [ Requires Acrobat ]
- Wikipedia – Nitrite
- EPA – Nutrient Pollution in Water
- EWG – Effects of Farming Pollution on Drinking Water Study [ Requires Acrobat ]
The foregoing information was compiled from the the links listed above.